Water, Ice, Hydrate
Water and ice are indispensable in our daily lives. We will clarify the mechanism of how these anomalous properties arise.
Theory and Simulation
We use statistical mechanics theory and computer simulation together to investigate phenomena invisible to experiment and to predict the properties of materials prior to experimentation.
Mysteries around You
Why does ice float on water? Why don’t the oceans expand as the Earth warms? Why are methane hydrates found on the ocean floor? Simple questions are not always easy to answer.
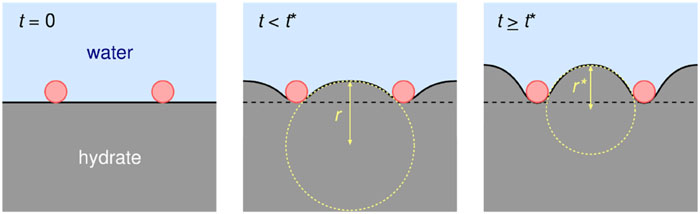
Statistical mechanical theory and computational study on thermodynamic stability of clathrate hydrates
A new paper from our group has been published. Clathrate hydrates are non-stoichiometric inclusion compounds with critical relevance to energy resources and CO2 sequestration, formed by guest molecules encapsulated in water cages. This perspective overviews the synergistic progress achieved through statistical mechanics and molecular simulation with intermolecular potential models in three key areas: thermodynamic stability, structural polymorphism, and dynamic processes. Theoretical estimation of its stability, originated from the van der Waals and Platteeuw theory, has been greatly improved by revisions accounting for constant pressure conditions, multiple occupancy, and host–guest coupling, enabling accurate prediction of multi-phase coexistence.
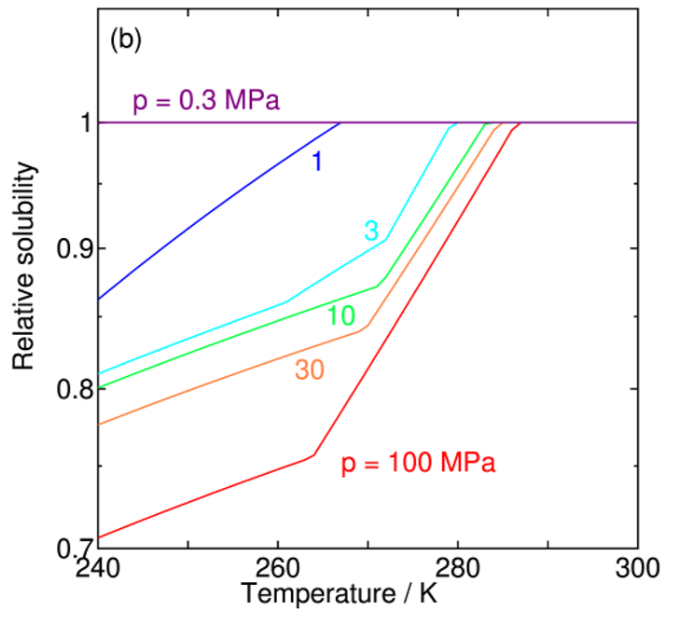
Solubility of Water in Carbon Dioxide
A new paper from our group has been published. This paper investigates the solubility of water in liquid carbon dioxide (CO2) under conditions where water or CO2 hydrate (clathrate hydrate) coexists, using theoretical calculations. The main focus is to quantitatively evaluate the reduction in solubility caused by hydrate formation under low-temperature and high-pressure conditions, and to clarify its temperature and pressure dependencies. This research provides valuable insights into thermodynamic properties related to practical challenges in large-scale CO2 transport, such as pipeline blockage and corrosion in carbon capture and storage (CCS).
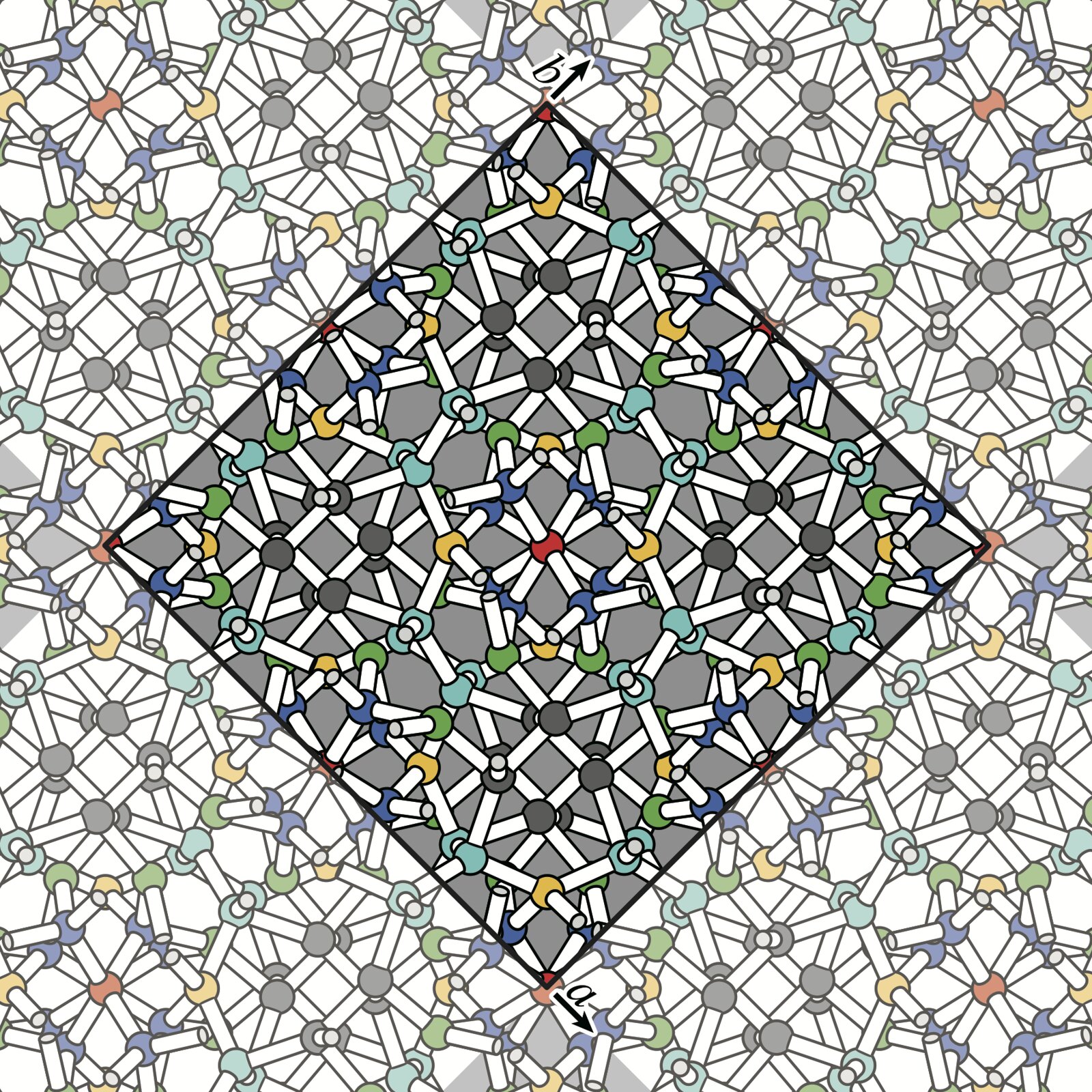
Ice T2 has been synthesized! (University of Tokyo)
More than 20 different crystal structures of ice have been discovered across various temperature and pressure ranges. This is considered an unusually large number of polymorphs for a pure substance. Of these, 12 were discovered by the 20th century, and the remaining 8 have been found since the 21st century, with the pace of discovery remarkably accelerating. Following the synthesis of plastic ice reported at the beginning of this year, there has been a report of the successful synthesis of ice T2, which our group predicted in 2018.
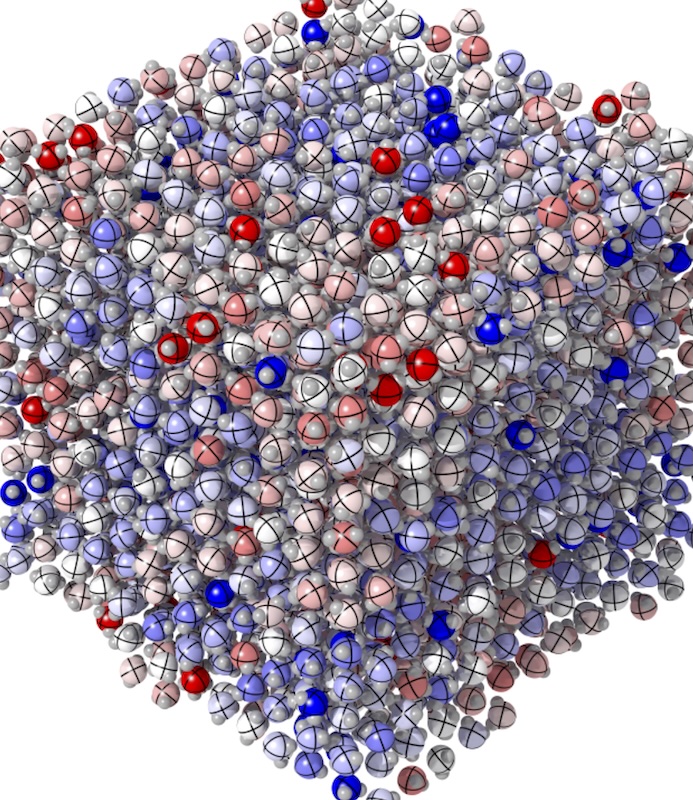
Plastic ice has been synthesized! (University of Rome)
The existence of plastic ice (plastic crystalline ice), predicted by the Tanaka group (the predecessor of our current laboratory) in 2008, has apparently been confirmed. Ice VII, which forms under ultra-high pressure, is said to be extremely hard. However, when heated, before the crystal structure collapses entirely, it transforms into a very soft crystal (barely maintaining its structural integrity) where water molecules can rotate freely. This state is called plastic ice (plastic crystalline ice).
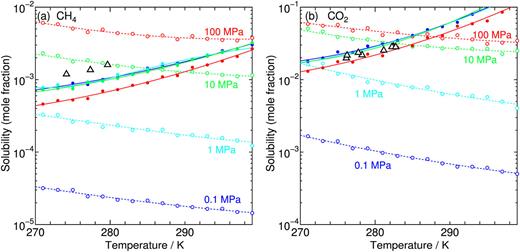
On the phase behaviors of CH4–CO2 binary clathrate hydrates`:` Equilibrium with aqueous phase
A new paper from our research group has been published. We explore the solubilities of guest CH4 and/or CO2 in the aqueous state coexisting with the corresponding hydrate. The equilibrium conditions are estimated by calculating the chemical potentials of water and guest species in the hydrate on the basis of a statistical mechanical theory using pairwise intermolecular potentials. This requires the least computational cost while covering a wide range of temperature, pressure, and composition of guest species, even for the binary hydrate.
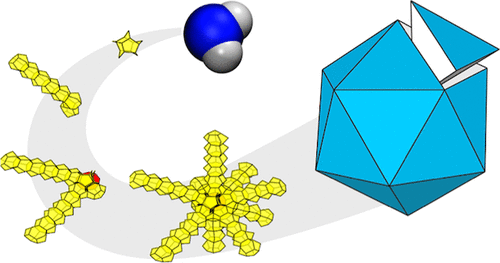
Emergence of the pentagonal ice nanocrystal
A new paper from our research group has been published. Press Release (in Japanese) Multitwinned nanocrystals are commonly found in substances that preferentially adopt tetrahedral local arrangements, but not yet in water crystals. Ice nanocrystals are pivotal in cloud microphysics, and their surfaces become increasingly prominent in determining structure as crystal size decreases. Nevertheless, discussions on nanocrystal structures have predominantly centered on ice polymorphs observed in bulk: hexagonal (Ih), cubic (Ic), and stacking-disordered (Isd) ices.
