Cage occupancy and dissociation enthalpy of hydrocarbon hydrates
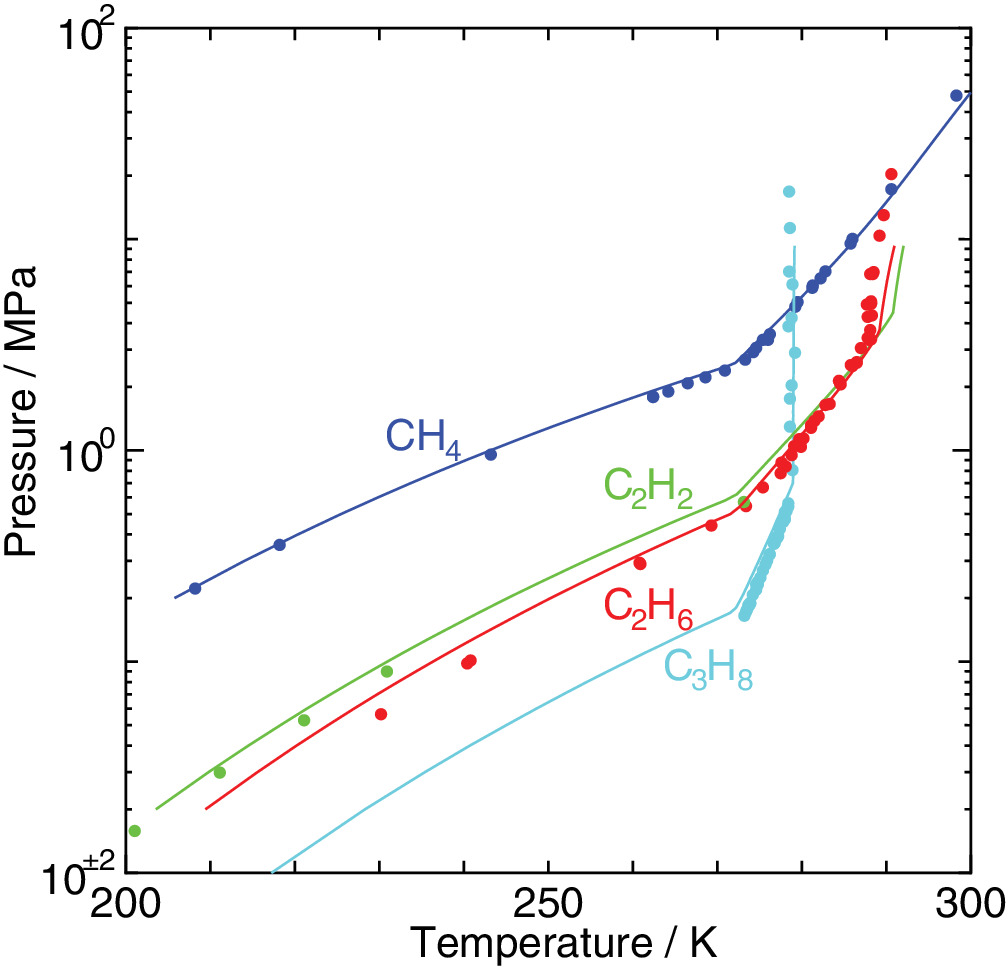
An elaborated statistical mechanical theory on clathrate hydrates is applied to exploration of their phase equilibria and dissociation enthalpies. The experimental dissociation pressures of methane, ethane, acetylene, and propane hydrates are well recovered by the method we have proposed. We estimate water/hydrate and hydrate/guest two‐phase coexisting conditions in the temperature, pressure, and composition space in addition to three‐phase equilibrium conditions. It is shown that the occupancy of guest molecules and the two‐phase boundaries in the phase diagram vary depending sensitively on its size. Enthalpy components arising from the host and guest interactions are separately calculated from the temperature dependence of the corresponding free energy values. This enables to evaluate the dissociation enthalpy at any stable and metastable thermodynamic state taking account of the phase transition in the coexisting phase such as melting of ice, notably that along the three‐phase equilibrium line.
Tanaka, H, Yagasaki, T, Matsumoto, M., Cage occupancy and dissociation enthalpy of hydrocarbon hydrates., AIChE J. 2020;e17009. https://doi.org/10.1002/aic.17009
