On the phase behaviors of CH4–CO2 binary clathrate hydrates:Two-phase and three-phase coexistences
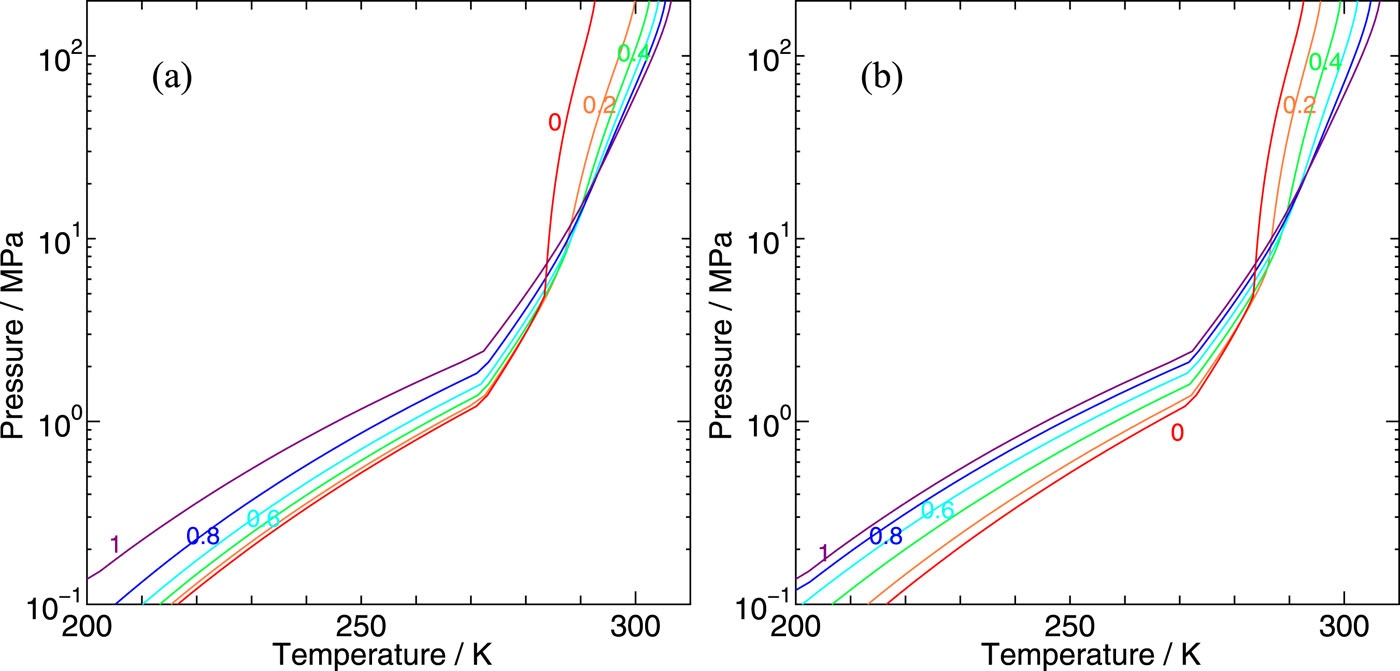
We develop a statistical mechanical theory on clathrate hydrates in order to explore the phase behaviors of clathrate hydrates containing two kinds of guest species and apply it to CH4–CO2 binary hydrates. The two boundaries separating water and hydrate and hydrate and guest fluid mixtures are estimated, which are extended to the lower temperature and the higher pressure region far distant from the three-phase coexisting conditions. The chemical potentials of individual guest components can be calculated from free energies of cage occupations, which are available from intermolecular interactions between host water and guest molecules. This allows us to derive all thermodynamic properties pertinent to the phase behaviors in the whole space of thermodynamic variables of temperature, pressure, and guest compositions. It is found that the phase boundaries of CH4–CO2 binary hydrates with water and with fluid mixtures locate between simple CH4 and CO2 hydrates, but the composition ratios of CH4 guests in hydrates are disproportional to those in fluid mixtures. Such differences arise from the affinities of each guest species to the large and small cages of CS-I hydrates and significantly affect occupation of each cage type, which results in a deviation of the guest composition in hydrates from that in fluid on the two-phase equilibrium conditions. The present method provides a basis for the evaluation of the efficiency of the guest CH4 replacement to CO2 at the thermodynamic limit.
Tanaka, H., Matsumoto, M. & Yagasaki, T. On the phase behaviors of CH4-CO2 binary clathrate hydrates: Two-phase and three-phase coexistences. J. Chem. Phys. 158, (2023) http://dx.doi.org/10.1063/5.0155143
