Stability mechanism of crystalline CO2 and Xe
A new paper from our research group has been published.
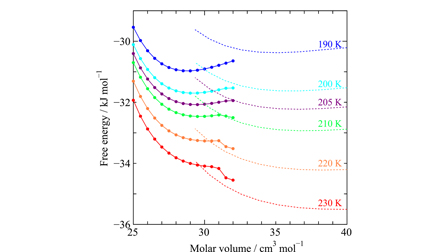
We explore the phase behaviors of simple molecular crystals in order to investigate the molecular basis of the stability mechanism relative to their liquid counterparts. The free energies of the face centered cubic crystals of Xe and CO2 are calculated as a collection of oscillators, and those of the liquids are from an equation of state via molecular dynamics simulations. The vibrational free energy in the solid is separated into the harmonic and anharmonic terms. The harmonic free energies decrease harshly with the expansion of the volume manifested as the large positive Grüneisen parameters, but the anharmonic free energies are positive and increase with volume, both of which originate from the deviation of the potential surface from the parabolic curve. The anharmonic free energies, though less significant in magnitude and destabilize the solids thermodynamically, serve to enhance their mechanical stability. The solid–liquid phase boundaries cannot be settled correctly without the exquisite balance between the two opposing contributions. A sharp contrast regarding the solid free energy is found in low-pressure ice, where the harmonic free energy does not decrease monotonically with volume and its anharmonic free energy is negative.
- Tanaka, H. et al. Stability mechanism of crystalline CO2 and Xe. J. Chem. Phys. 161, (2024).
- DOI:10.1063/5.0223879
