Emergence of the pentagonal ice nanocrystal
A new paper from our research group has been published.
- Press Release (in Japanese)
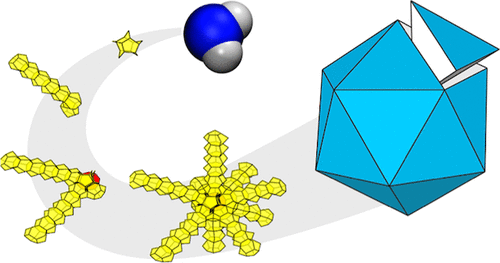
Multitwinned nanocrystals are commonly found in substances that preferentially adopt tetrahedral local arrangements, but not yet in water crystals. Ice nanocrystals are pivotal in cloud microphysics, and their surfaces become increasingly prominent in determining structure as crystal size decreases. Nevertheless, discussions on nanocrystal structures have predominantly centered on ice polymorphs observed in bulk: hexagonal (Ih), cubic (Ic), and stacking-disordered (Isd) ices. Here, we demonstrate, through molecular dynamics (MD) simulations, that decahedral and icosahedral nanocrystals form from liquid water droplets of a few nanometers in size without violating the ice rule. The brute force spontaneous crystallization is conducted using the mW model, and the thermodynamic stability is examined using the TIP4P/Ice model. During the crystallization process, the formation of twin boundaries precedes the emergence of centers exhibiting 5-fold and icosahedral symmetry. The free energy calculation suggests the icosahedron has comparable stability with ice Ih nanocrystal. The frequent occurrence of these unreported ice nanocrystals aligns with the fact that natural polycrystalline snow crystals predominantly display a 70.5-degree angle between the Ih c-axes of adjacent branches. Moreover, we show that the formation of multitwinned ice nanocrystals is enhanced within a fullerene, providing a potential avenue for experimental observations.
- Zhang, X., Matsumoto, M., Zhang, Z. & Mochizuki, K. Multitwinned ice nanocrystals. ACS Nano (2024) .
- DOI:10.1021/acsnano.4c07226
