Solubility of Water in Carbon Dioxide
A new paper from our group has been published.
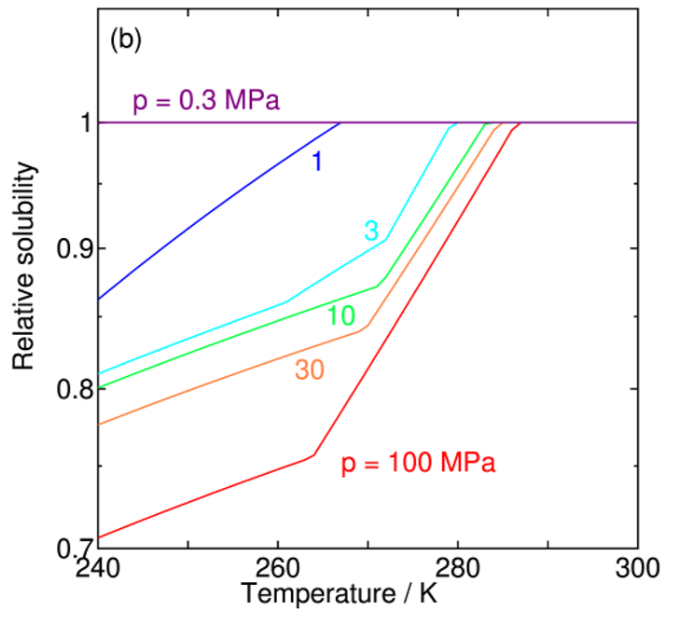
This paper investigates the solubility of water in liquid carbon dioxide (CO2) under conditions where water or CO2 hydrate (clathrate hydrate) coexists, using theoretical calculations. The main focus is to quantitatively evaluate the reduction in solubility caused by hydrate formation under low-temperature and high-pressure conditions, and to clarify its temperature and pressure dependencies. This research provides valuable insights into thermodynamic properties related to practical challenges in large-scale CO2 transport, such as pipeline blockage and corrosion in carbon capture and storage (CCS). The research team has successfully reproduced experimentally observed solubility curves with high accuracy by introducing corrections to the self-polarization energy of specific water potential models through chemical potential calculations.
- Tanaka, H. et al. The solubilities of water in liquid CO2 coexisting with water or hydrate. J. Chem. Phys. 163, 124504 (2025)
- DOI:10.1063/5.0294608
