carbon dioxide
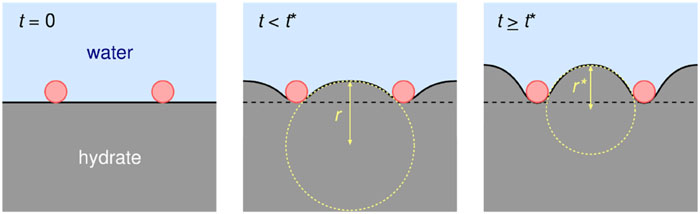
Statistical mechanical theory and computational study on thermodynamic stability of clathrate hydrates
A new paper from our group has been published. Clathrate hydrates are non-stoichiometric inclusion compounds with critical relevance to energy resources and CO2 sequestration, formed by guest molecules encapsulated in water cages. This perspective overviews the synergistic progress achieved through statistical mechanics and molecular simulation with intermolecular potential models in three key areas: thermodynamic stability, structural polymorphism, and dynamic processes. Theoretical estimation of its stability, originated from the van der Waals and Platteeuw theory, has been greatly improved by revisions accounting for constant pressure conditions, multiple occupancy, and host–guest coupling, enabling accurate prediction of multi-phase coexistence.
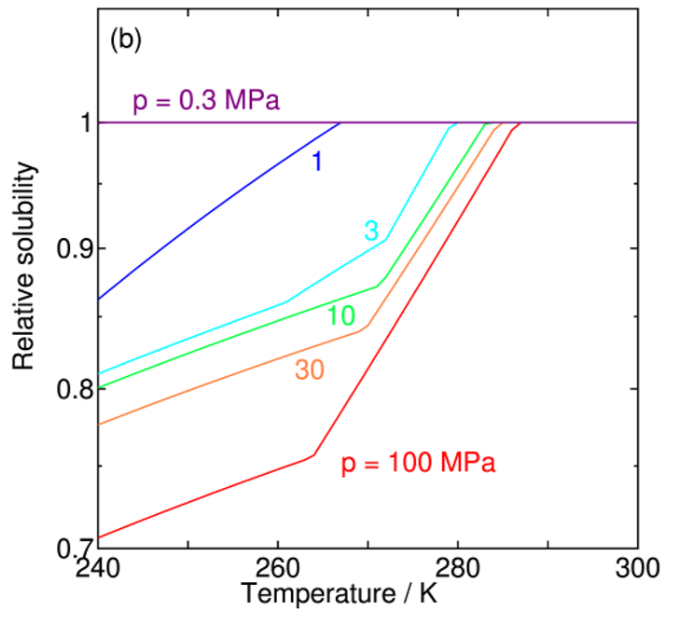
Solubility of Water in Carbon Dioxide
A new paper from our group has been published. This paper investigates the solubility of water in liquid carbon dioxide (CO2) under conditions where water or CO2 hydrate (clathrate hydrate) coexists, using theoretical calculations. The main focus is to quantitatively evaluate the reduction in solubility caused by hydrate formation under low-temperature and high-pressure conditions, and to clarify its temperature and pressure dependencies. This research provides valuable insights into thermodynamic properties related to practical challenges in large-scale CO2 transport, such as pipeline blockage and corrosion in carbon capture and storage (CCS).
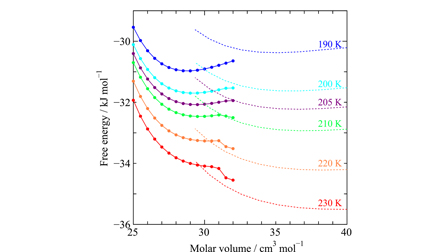
Stability mechanism of crystalline CO2 and Xe
A new paper from our research group has been published. We explore the phase behaviors of simple molecular crystals in order to investigate the molecular basis of the stability mechanism relative to their liquid counterparts. The free energies of the face centered cubic crystals of Xe and CO2 are calculated as a collection of oscillators, and those of the liquids are from an equation of state via molecular dynamics simulations. The vibrational free energy in the solid is separated into the harmonic and anharmonic terms.
