clathrate hydrate
Statistical mechanical theory and computational study on thermodynamic stability of clathrate hydrates
A new paper from our group has been published. Clathrate hydrates are non-stoichiometric inclusion compounds with critical relevance to energy resources and CO2 sequestration, formed by guest molecules encapsulated in water cages. This perspective overviews the synergistic progress achieved through statistical mechanics and molecular simulation with intermolecular potential models in three key areas: thermodynamic stability, structural polymorphism, and dynamic processes. Theoretical estimation of its stability, originated from the van der Waals and Platteeuw theory, has been greatly improved by revisions accounting for constant pressure conditions, multiple occupancy, and host–guest coupling, enabling accurate prediction of multi-phase coexistence.
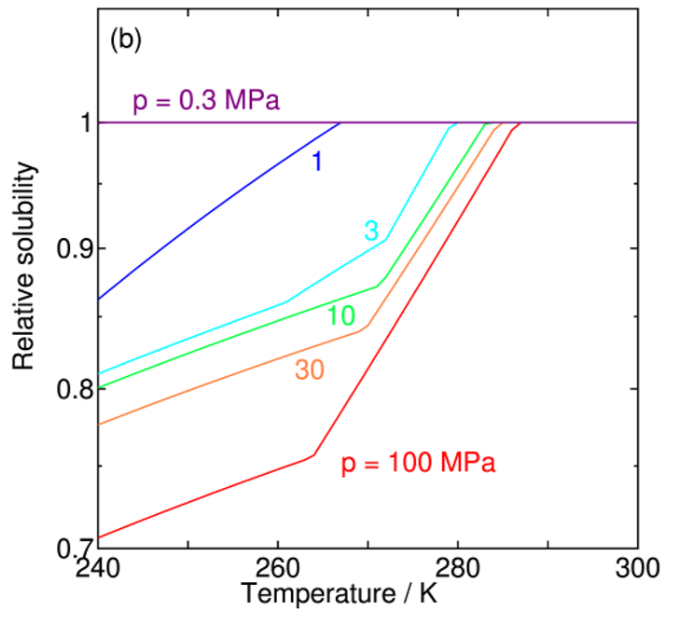
Solubility of Water in Carbon Dioxide
A new paper from our group has been published. This paper investigates the solubility of water in liquid carbon dioxide (CO2) under conditions where water or CO2 hydrate (clathrate hydrate) coexists, using theoretical calculations. The main focus is to quantitatively evaluate the reduction in solubility caused by hydrate formation under low-temperature and high-pressure conditions, and to clarify its temperature and pressure dependencies. This research provides valuable insights into thermodynamic properties related to practical challenges in large-scale CO2 transport, such as pipeline blockage and corrosion in carbon capture and storage (CCS).
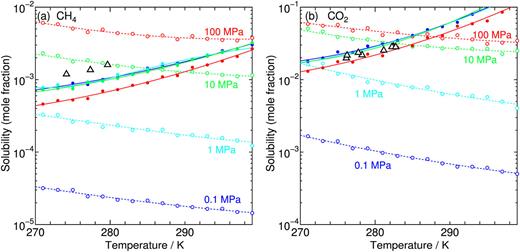
On the phase behaviors of CH4–CO2 binary clathrate hydrates`:` Equilibrium with aqueous phase
A new paper from our research group has been published. We explore the solubilities of guest CH4 and/or CO2 in the aqueous state coexisting with the corresponding hydrate. The equilibrium conditions are estimated by calculating the chemical potentials of water and guest species in the hydrate on the basis of a statistical mechanical theory using pairwise intermolecular potentials. This requires the least computational cost while covering a wide range of temperature, pressure, and composition of guest species, even for the binary hydrate.
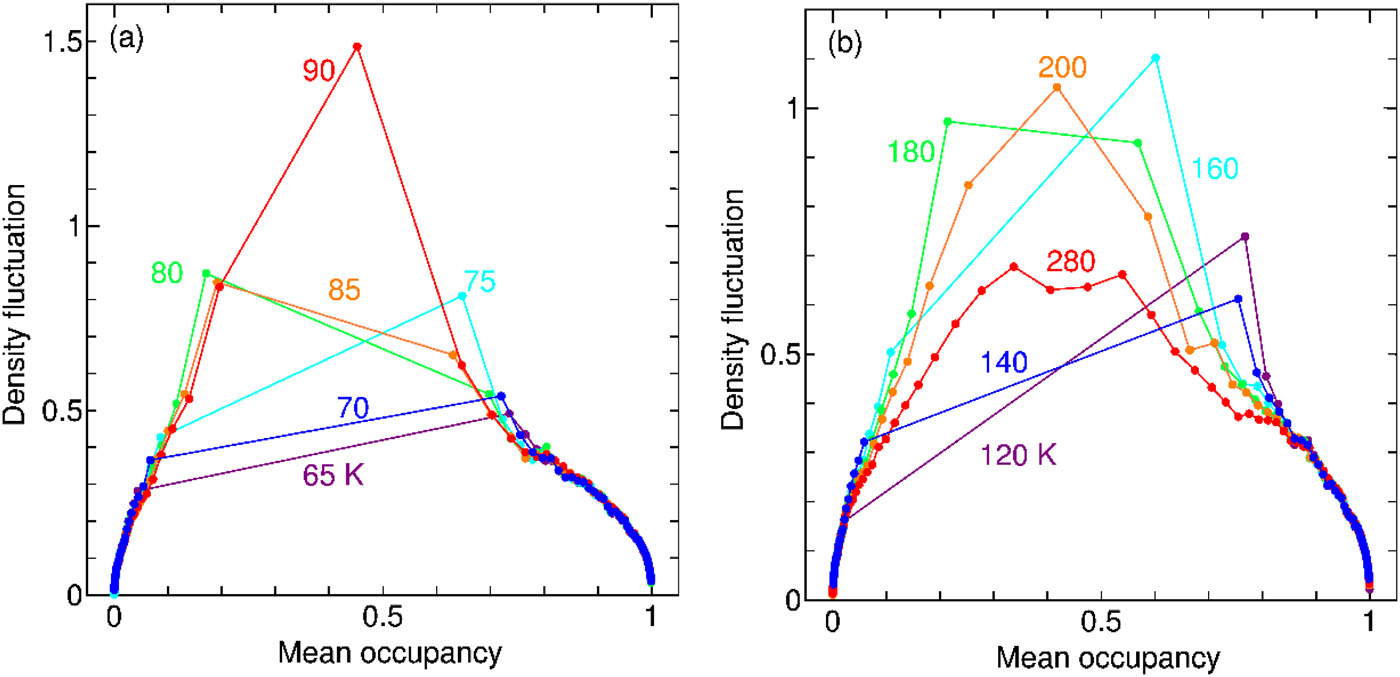
Cage occupancies of CH4, CO2, and Xe hydrates:Mean field theory and grandcanonical Monte Carlo simulations
A new paper from our research group has been published. We propose a statistical mechanical theory for the thermodynamic stability of clathrate hydrates, considering the influence of the guest–guest interaction on the occupancies of the cages. A mean field approximation is developed to examine the magnitude of the influence. Our new method works remarkably well, which is manifested by two sorts of grandcanonical Monte Carlo (GCMC) simulations. One is full GCMC, and the other is designed in the present study for clathrate hydrates, called lattice-GCMC, in which each guest can be adsorbed at one of the centers of the cage.
Efficiency and energy balance for substitution of CH4 in clathrate hydrates with CO2 under multiple-phase coexisting conditions
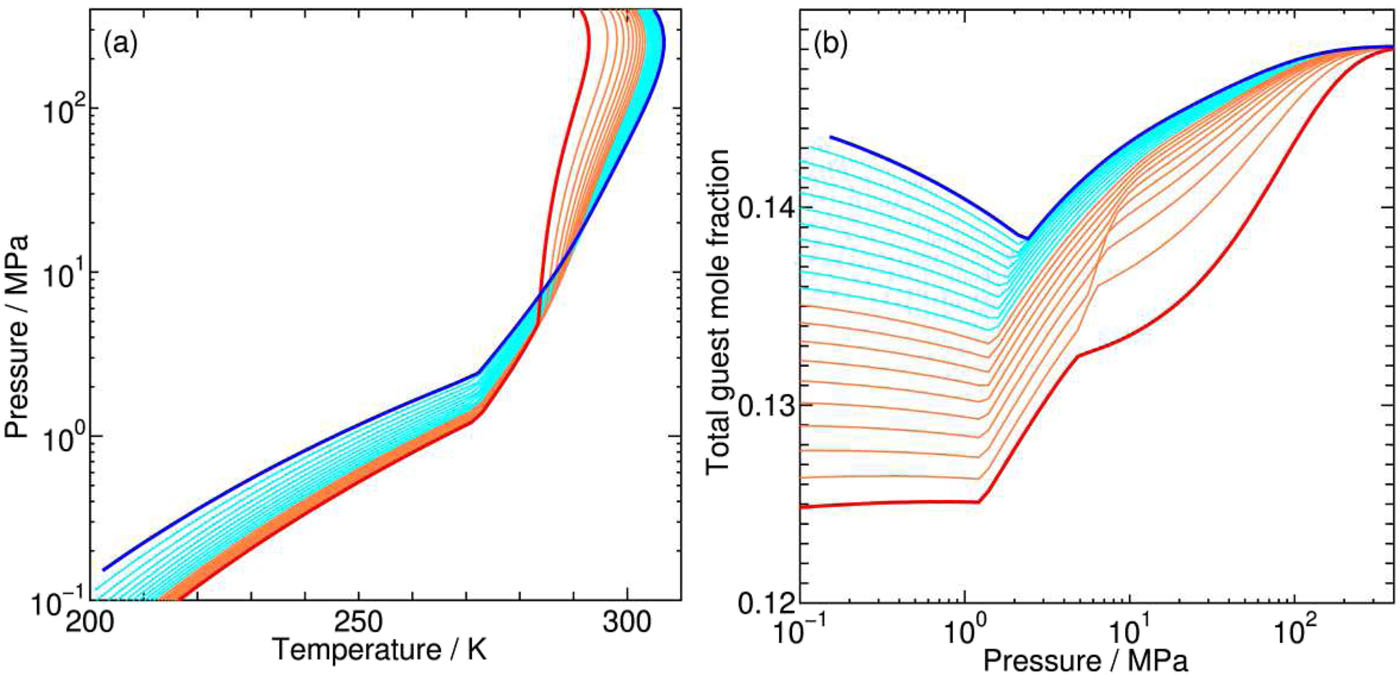
Many experimental and theoretical studies on CH4–CO2 hydrates have been performed aiming at the extraction of CH4 as a relatively clean energy resource and concurrent sequestration of CO2. However, vague or insufficient characterization of the environmental conditions prevents us from a comprehensive understanding of even equilibrium properties of CH4–CO2 hydrates for this substitution. We propose possible reaction schemes for the substitution, paying special attention to the coexisting phases, the aqueous and/or the fluid, where CO2 is supplied from and CH4 is transferred to.
On the phase behaviors of CH4–CO2 binary clathrate hydrates:Two-phase and three-phase coexistences
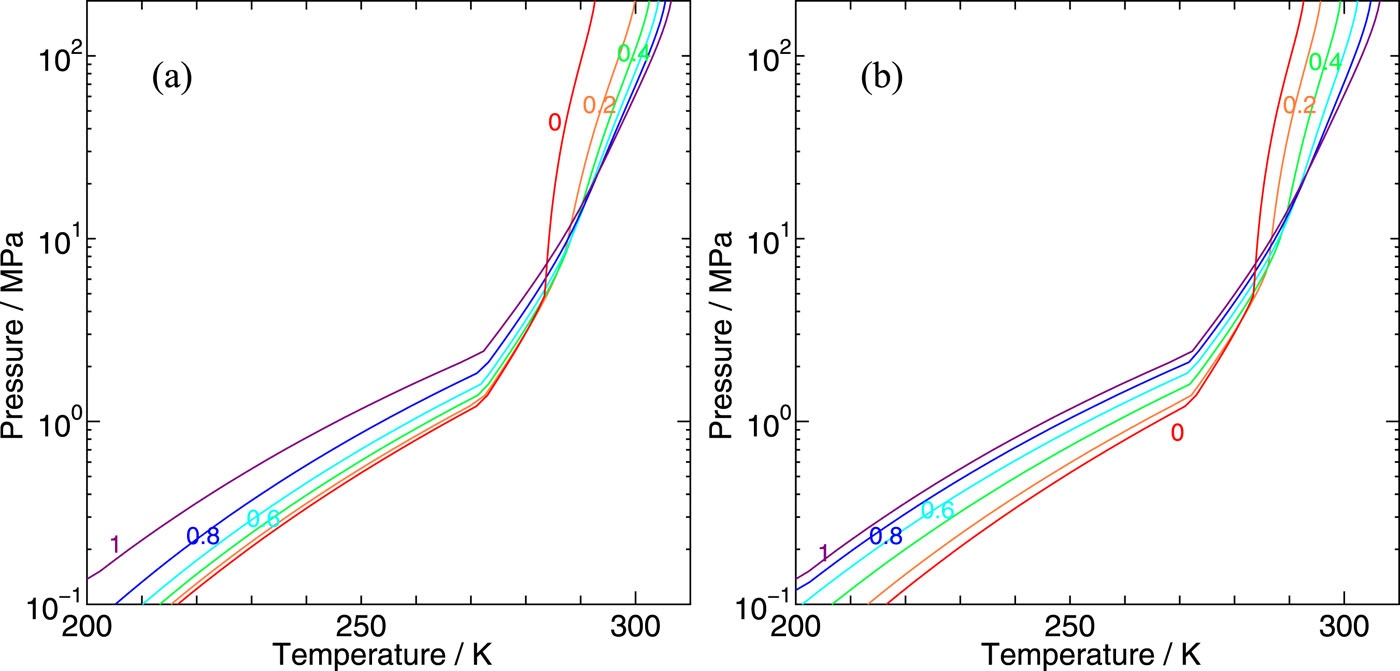
We develop a statistical mechanical theory on clathrate hydrates in order to explore the phase behaviors of clathrate hydrates containing two kinds of guest species and apply it to CH4–CO2 binary hydrates. The two boundaries separating water and hydrate and hydrate and guest fluid mixtures are estimated, which are extended to the lower temperature and the higher pressure region far distant from the three-phase coexisting conditions. The chemical potentials of individual guest components can be calculated from free energies of cage occupations, which are available from intermolecular interactions between host water and guest molecules.
Structure Selectivity of Mixed Gas Hydrates and Group 14 Clathrates
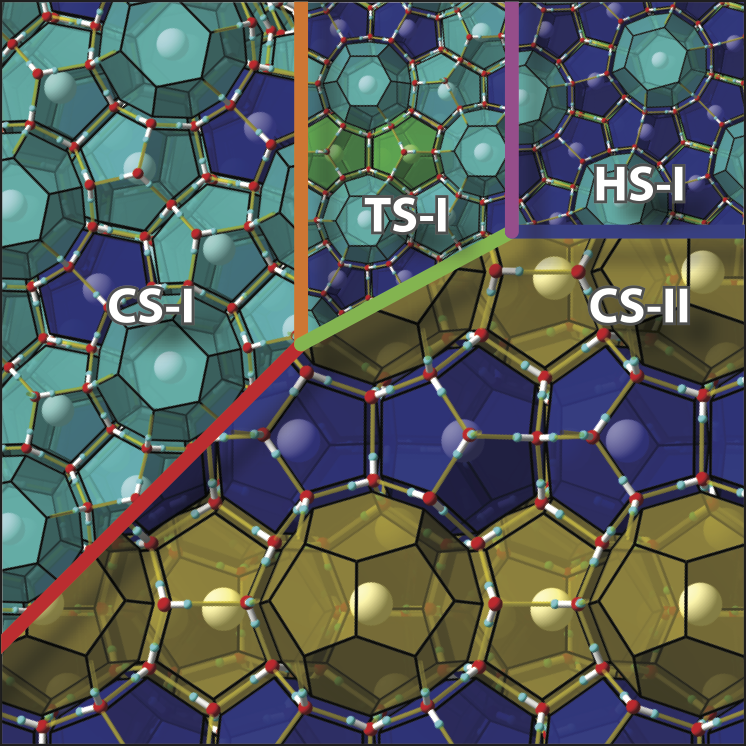
A new paper from our group has been published. In a previous paper, we examined the regularity with which the crystal structure of inclusion hydrates is chosen. We applied that approach to a new mixed gas inclusion hydrates and group 14 clathrate compounds. In the former, we presented an overarching explanation for why mixing gases may change the crystal structure. Adding just a few molecules of a certain type may significantly change the crystal structure.
Cage occupancy and dissociation enthalpy of hydrocarbon hydrates
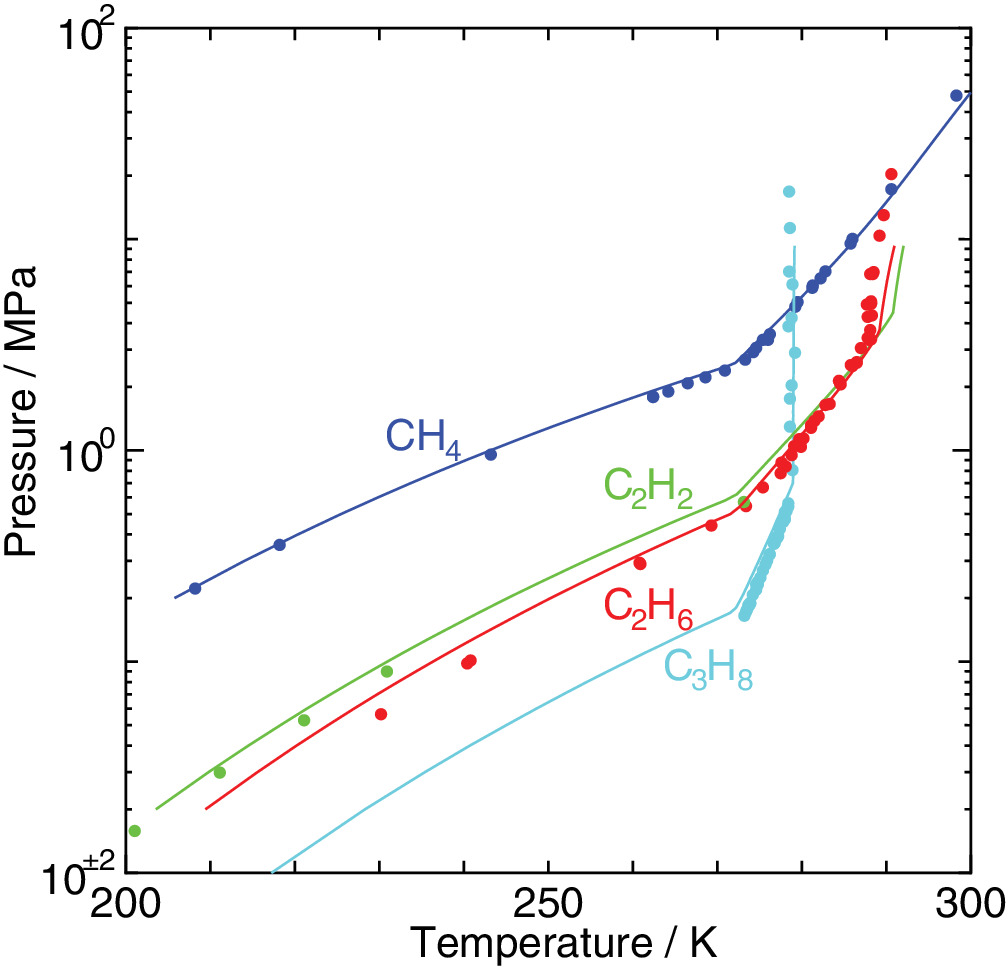
An elaborated statistical mechanical theory on clathrate hydrates is applied to exploration of their phase equilibria and dissociation enthalpies. The experimental dissociation pressures of methane, ethane, acetylene, and propane hydrates are well recovered by the method we have proposed. We estimate water/hydrate and hydrate/guest two‐phase coexisting conditions in the temperature, pressure, and composition space in addition to three‐phase equilibrium conditions. It is shown that the occupancy of guest molecules and the two‐phase boundaries in the phase diagram vary depending sensitively on its size.
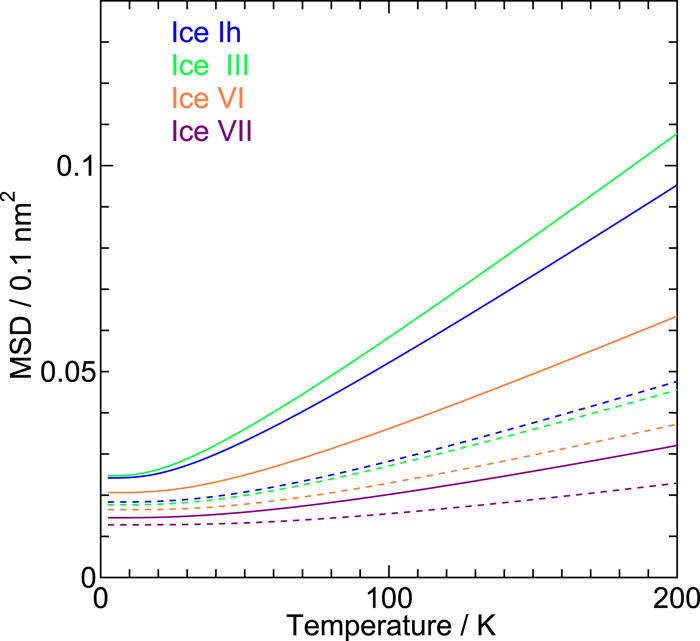
On the role of intermolecular vibrational motions for ice polymorphs II:\ Atomic vibrational amplitudes and localization of phonons in ordered and disordered ices
We investigate the vibrational amplitudes and the degree of the phonon localization in 19 ice forms, both crystalline and amorphous, by a quasi-harmonic approximation with a reliable classical intermolecular interaction model for water. The amplitude in the low pressure ices increases with compression, while the opposite trend is observed in the medium and high pressure ices. The amplitude of the oxygen atom does not differ from that of hydrogen in low pressure ices apart from the contribution from the zero-point vibrations.
