hydrogen bond
Novel Algorithm to Generate Hydrogen-Disordered Ice Structures
A new paper from our group has been published. There is a growing demand for computer simulations of ice, which is composed of a large number of molecules, to study the behavior of molecules dissolved in small amounts in ice and to search for new crystal structures. Unlike ordinary crystals, the water molecules in ice crystals are not oriented in the same way. In order to handle ice in computer simulations, it is necessary to generate crystal structures with randomized molecular orientations in an appropriate manner.
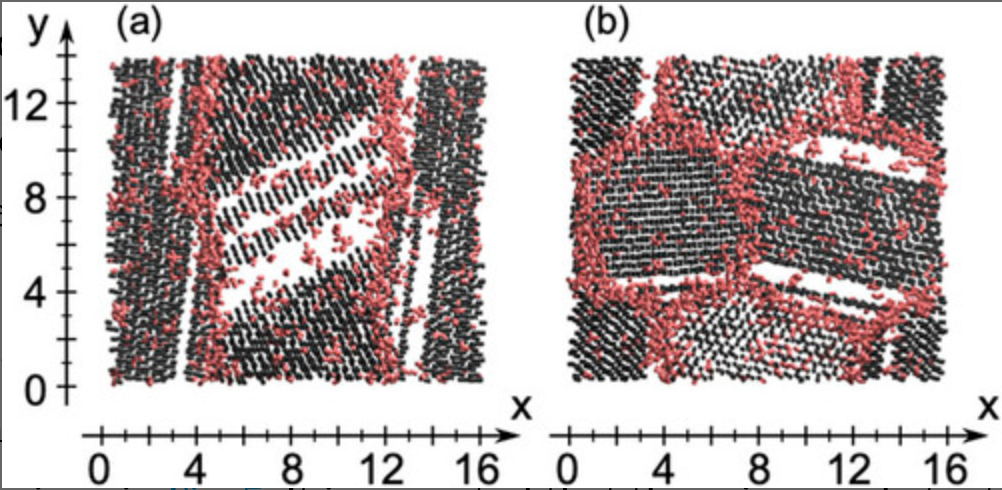
Molecular dynamics study of grain boundaries and triple junctions in ice
We perform classical molecular dynamics simulations of polycrystalline ice at 250 K using the TIP4P/Ice model. The structures of polycrystalline ice are prepared by growing ice particles in supercooled water. An order parameter developed recently is used to characterize local structures in terms of the liquid–liquid phase transition scenario. It is shown that the grain boundaries and triple junctions in ice are structurally similar to low-density liquid water in which most water molecules form four hydrogen bonds and the O–O–O angles deviate from the tetrahedral angle of 109.
