paper2020
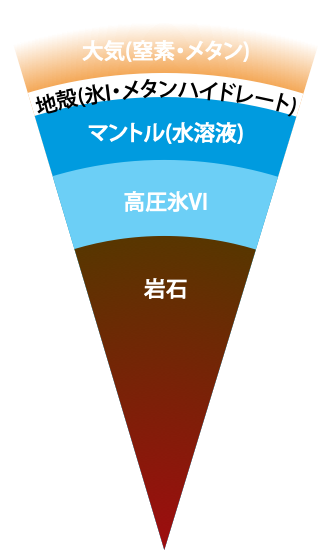
On the Occurrence of Clathrate Hydrates in Extreme Conditions:\ Dissociation Pressures and Occupancies at Cryogenic Temperatures with Application to Planetary Systems
We investigate the thermodynamic stability of clathrate hydrates at cryogenic temperatures from the 0 K limit to 200 K in a wide range of pressures, covering the thermodynamic conditions of interstellar space and the surface of the hydrosphere in satellites. Our evaluation of the phase behaviors is performed by setting up quantum partition functions with variable pressures on the basis of a rigorous statistical mechanics theory that requires only the intermolecular interactions as input.
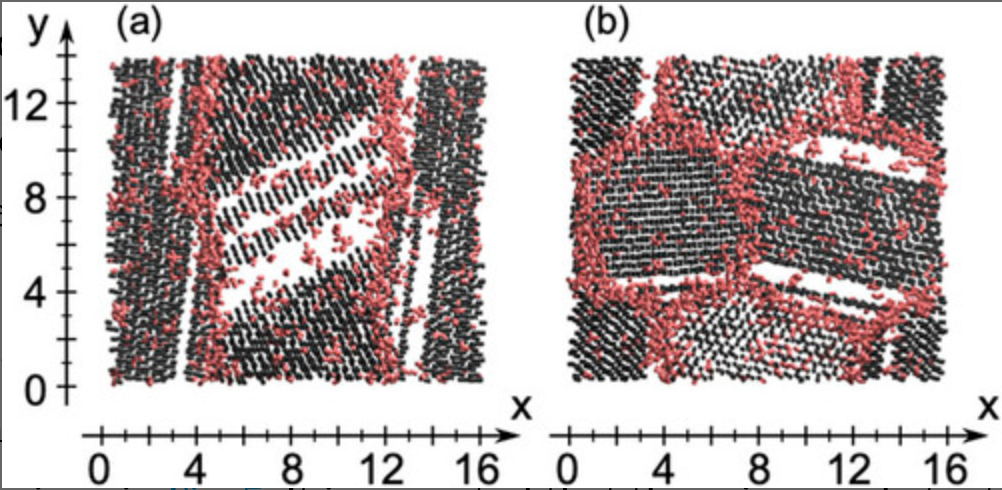
Molecular dynamics study of grain boundaries and triple junctions in ice
We perform classical molecular dynamics simulations of polycrystalline ice at 250 K using the TIP4P/Ice model. The structures of polycrystalline ice are prepared by growing ice particles in supercooled water. An order parameter developed recently is used to characterize local structures in terms of the liquid–liquid phase transition scenario. It is shown that the grain boundaries and triple junctions in ice are structurally similar to low-density liquid water in which most water molecules form four hydrogen bonds and the O–O–O angles deviate from the tetrahedral angle of 109.
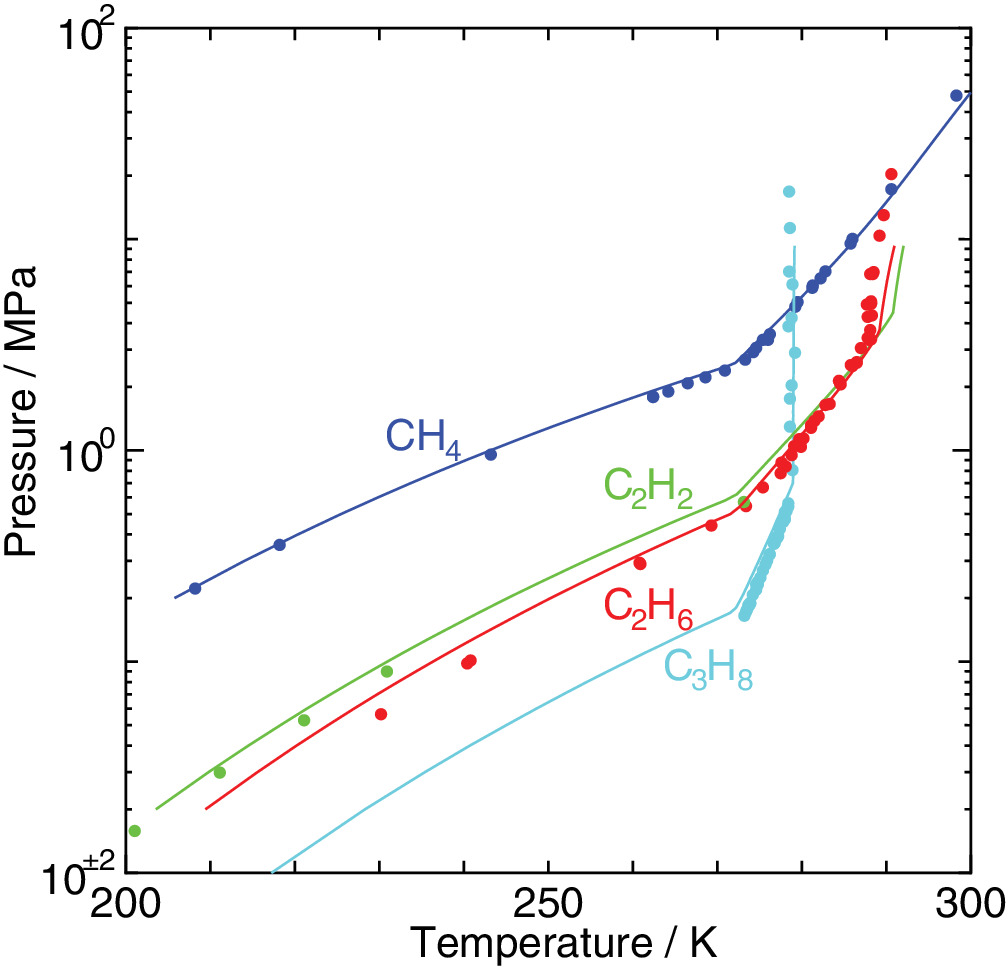
Cage occupancy and dissociation enthalpy of hydrocarbon hydrates
An elaborated statistical mechanical theory on clathrate hydrates is applied to exploration of their phase equilibria and dissociation enthalpies. The experimental dissociation pressures of methane, ethane, acetylene, and propane hydrates are well recovered by the method we have proposed. We estimate water/hydrate and hydrate/guest two‐phase coexisting conditions in the temperature, pressure, and composition space in addition to three‐phase equilibrium conditions. It is shown that the occupancy of guest molecules and the two‐phase boundaries in the phase diagram vary depending sensitively on its size.
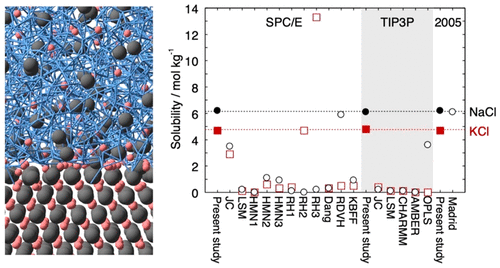
Lennard-Jones Parameters Determined to Reproduce the Solubility of ions
Most classical nonpolarizable ion potential models underestimate the solubility values of NaCl and KCl in water significantly. We determine Lennard-Jones parameters of Na+, K+, and Cl- that reproduce the solubility as well as the hydration free energy in dilute aqueous solutions for three water potential models, SPC/E, TIP3P, and TIP4P/2005. The ion–oxygen distance in the solution and the cation–anion distance in salt are also considered in the parametrization. In addition to the target properties, the hydration enthalpy, hydration entropy, self-diffusion coefficient, coordination number, lattice energy, enthalpy of solution, density, viscosity, and number of contact ion pairs are calculated for comparison with 17 frequently used or recently developed ion potential models.
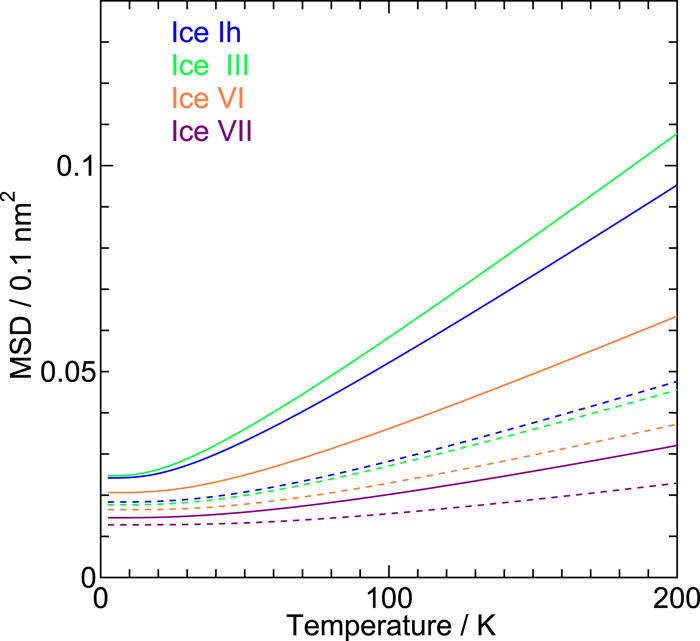
On the role of intermolecular vibrational motions for ice polymorphs II:\ Atomic vibrational amplitudes and localization of phonons in ordered and disordered ices
We investigate the vibrational amplitudes and the degree of the phonon localization in 19 ice forms, both crystalline and amorphous, by a quasi-harmonic approximation with a reliable classical intermolecular interaction model for water. The amplitude in the low pressure ices increases with compression, while the opposite trend is observed in the medium and high pressure ices. The amplitude of the oxygen atom does not differ from that of hydrogen in low pressure ices apart from the contribution from the zero-point vibrations.
