paper2021

On the role of intermolecular vibrational motions for ice polymorphs. III. Mode characteristics associated with negative thermal expansion.
It is well known as an unusual property of liquid water that when it is cooled down, it begins to expand at a temperature below 4 degree celsius. When it is cooled down to 0 degree, it becomes ice, and after that, its volume becomes smaller as it is cooled down. However, even after it becomes ice, if the temperature is kept very low, it begins to expand again at an absolute temperature of 60 K or lower.
On the anomalous homogeneity of hydrogen-disordered ice and its origin
In hydrogen-disordered ice, each water molecule is oriented in a different way, and the interaction between one water molecule and the surrounding water molecules can be attractive (low interaction energy) or repulsive (high interaction energy). The interaction between a water molecule and a large number of surrounding water molecules seems to be most influenced by the orientation of the water molecules in the immediate vicinity. However, calculations and experiments have shown that the interaction between a water molecule and all surrounding water molecules is almost the same regardless of whether the direction of the nearby water molecule is attractive or repulsive.
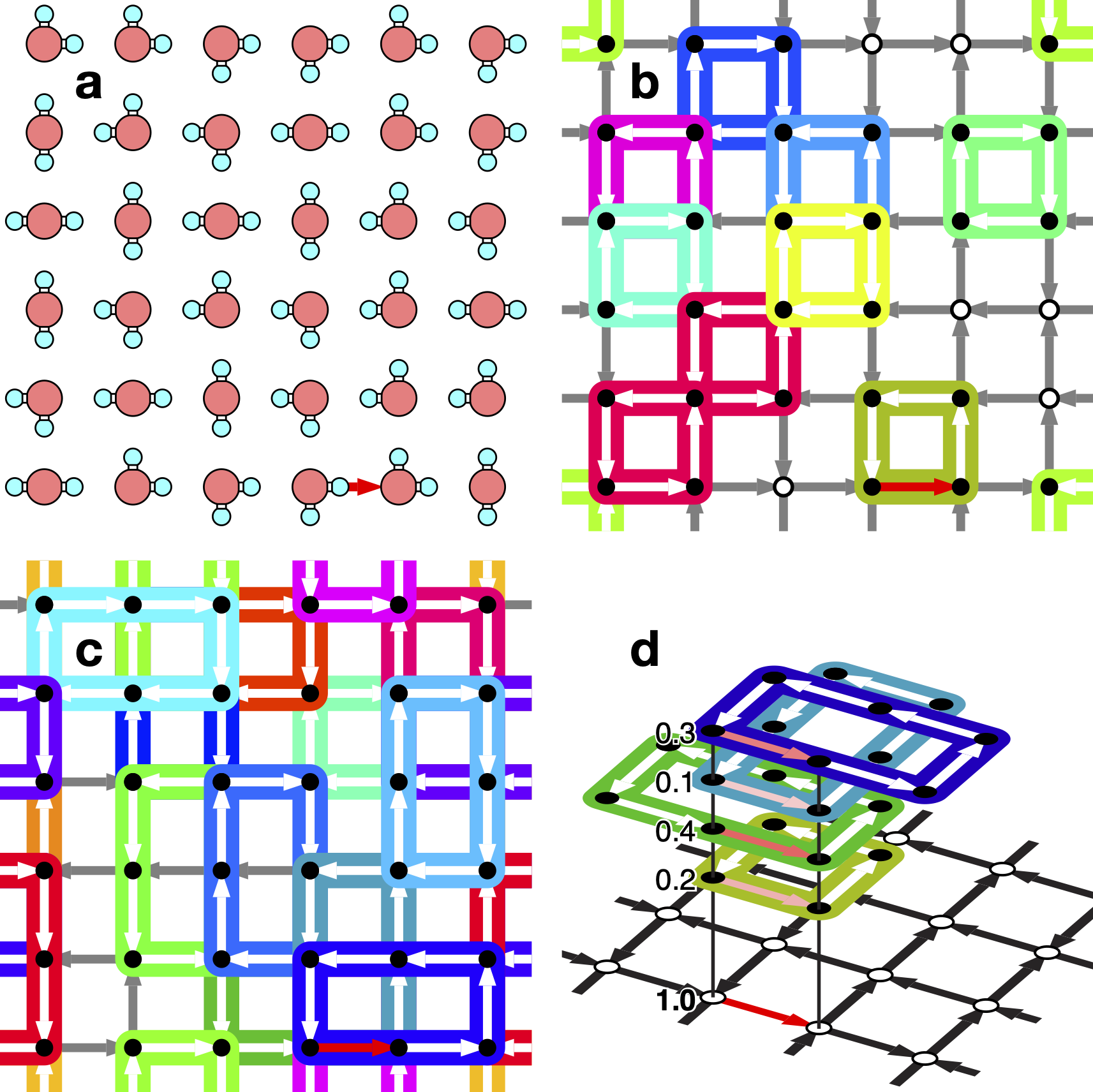
Novel Algorithm to Generate Hydrogen-Disordered Ice Structures
A new paper from our group has been published. There is a growing demand for computer simulations of ice, which is composed of a large number of molecules, to study the behavior of molecules dissolved in small amounts in ice and to search for new crystal structures. Unlike ordinary crystals, the water molecules in ice crystals are not oriented in the same way. In order to handle ice in computer simulations, it is necessary to generate crystal structures with randomized molecular orientations in an appropriate manner.
