paper2023
Efficiency and energy balance for substitution of CH4 in clathrate hydrates with CO2 under multiple-phase coexisting conditions
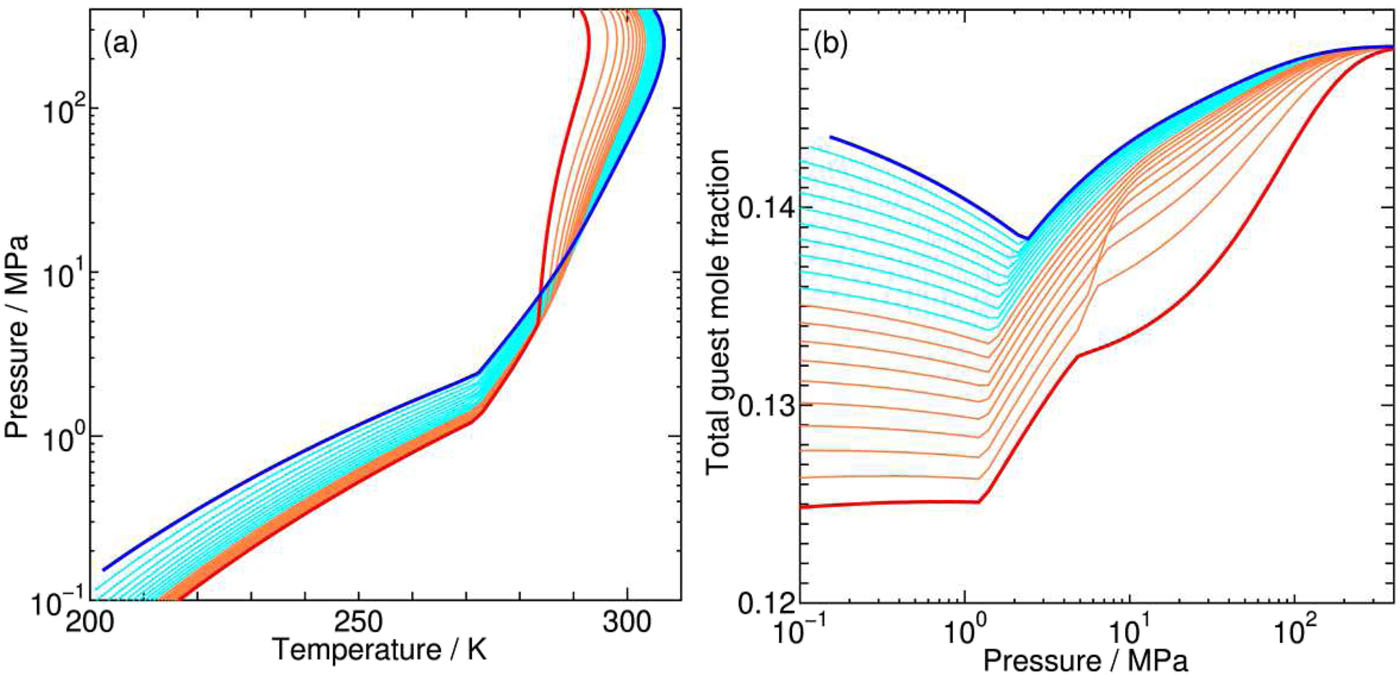
Many experimental and theoretical studies on CH4–CO2 hydrates have been performed aiming at the extraction of CH4 as a relatively clean energy resource and concurrent sequestration of CO2. However, vague or insufficient characterization of the environmental conditions prevents us from a comprehensive understanding of even equilibrium properties of CH4–CO2 hydrates for this substitution. We propose possible reaction schemes for the substitution, paying special attention to the coexisting phases, the aqueous and/or the fluid, where CO2 is supplied from and CH4 is transferred to.
On the phase behaviors of CH4–CO2 binary clathrate hydrates:Two-phase and three-phase coexistences
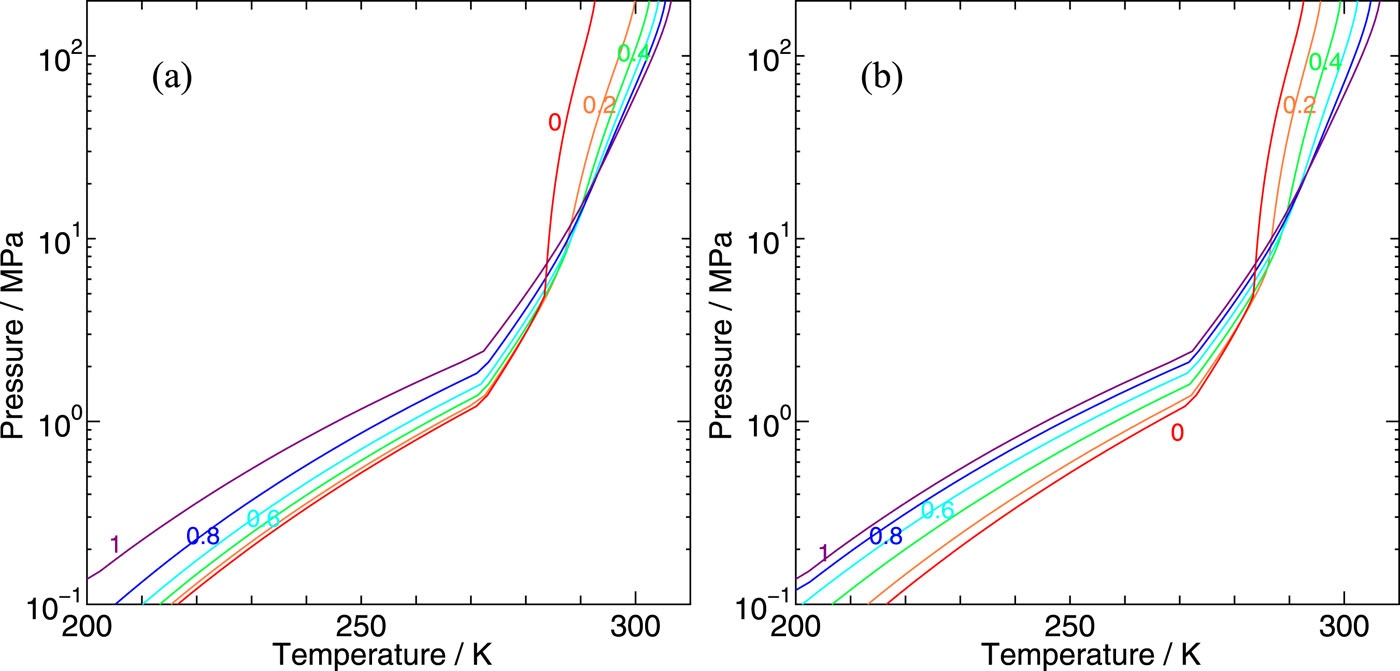
We develop a statistical mechanical theory on clathrate hydrates in order to explore the phase behaviors of clathrate hydrates containing two kinds of guest species and apply it to CH4–CO2 binary hydrates. The two boundaries separating water and hydrate and hydrate and guest fluid mixtures are estimated, which are extended to the lower temperature and the higher pressure region far distant from the three-phase coexisting conditions. The chemical potentials of individual guest components can be calculated from free energies of cage occupations, which are available from intermolecular interactions between host water and guest molecules.
