partition function
On the Occurrence of Clathrate Hydrates in Extreme Conditions:\ Dissociation Pressures and Occupancies at Cryogenic Temperatures with Application to Planetary Systems
We investigate the thermodynamic stability of clathrate hydrates at cryogenic temperatures from the 0 K limit to 200 K in a wide range of pressures, covering the thermodynamic conditions of interstellar space and the surface of the hydrosphere in satellites. Our evaluation of the phase behaviors is performed by setting up quantum partition functions with variable pressures on the basis of a rigorous statistical mechanics theory that requires only the intermolecular interactions as input.
Cage occupancy and dissociation enthalpy of hydrocarbon hydrates
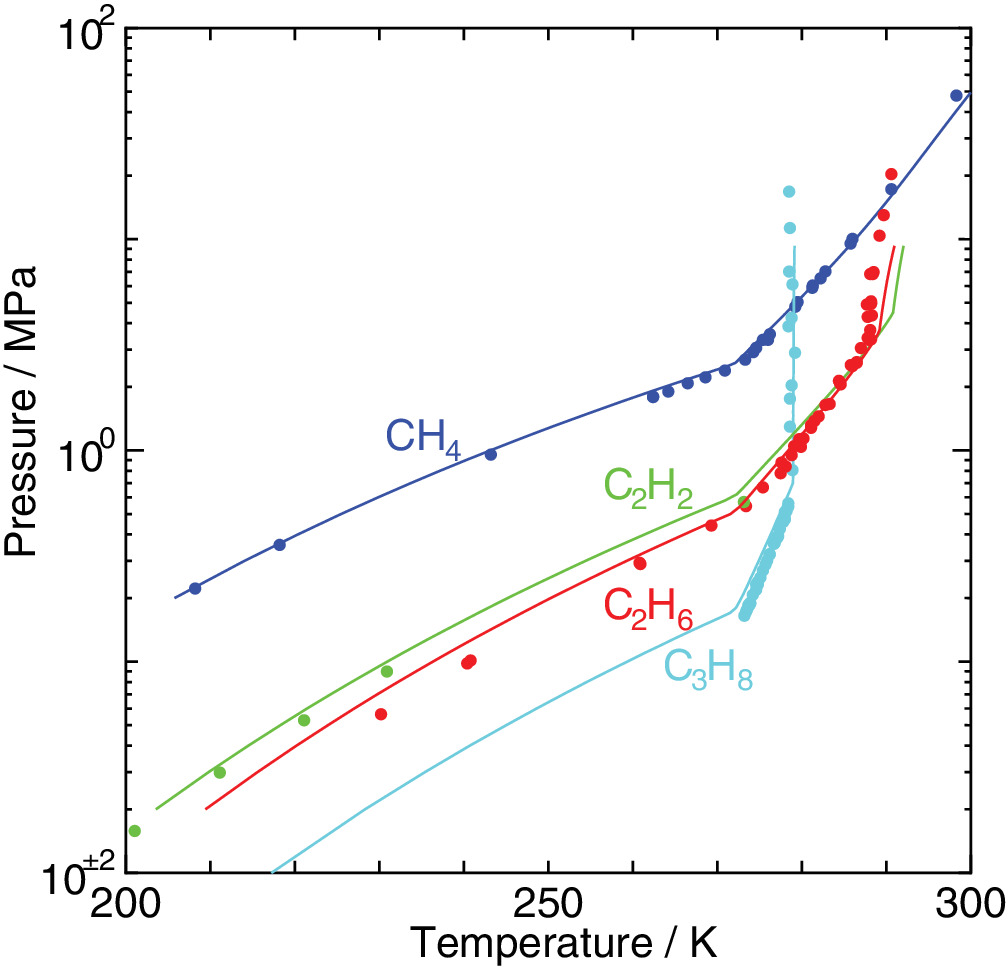
An elaborated statistical mechanical theory on clathrate hydrates is applied to exploration of their phase equilibria and dissociation enthalpies. The experimental dissociation pressures of methane, ethane, acetylene, and propane hydrates are well recovered by the method we have proposed. We estimate water/hydrate and hydrate/guest two‐phase coexisting conditions in the temperature, pressure, and composition space in addition to three‐phase equilibrium conditions. It is shown that the occupancy of guest molecules and the two‐phase boundaries in the phase diagram vary depending sensitively on its size.
On the role of intermolecular vibrational motions for ice polymorphs II:\ Atomic vibrational amplitudes and localization of phonons in ordered and disordered ices
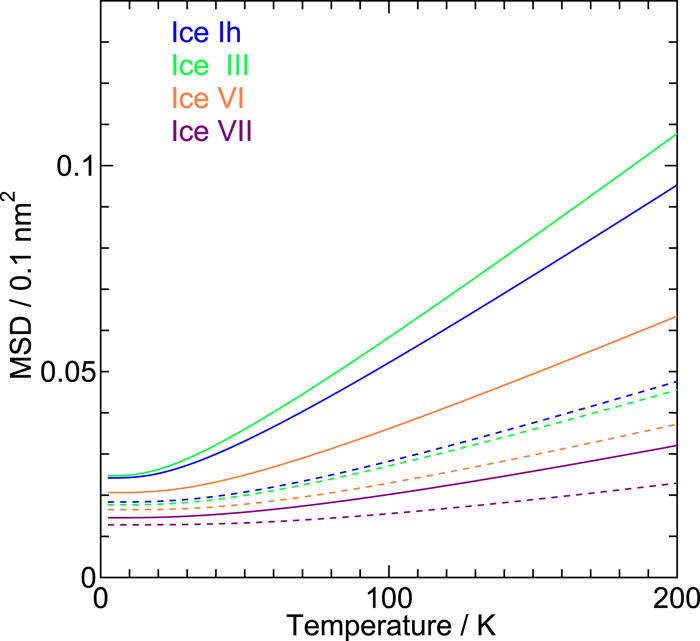
We investigate the vibrational amplitudes and the degree of the phonon localization in 19 ice forms, both crystalline and amorphous, by a quasi-harmonic approximation with a reliable classical intermolecular interaction model for water. The amplitude in the low pressure ices increases with compression, while the opposite trend is observed in the medium and high pressure ices. The amplitude of the oxygen atom does not differ from that of hydrogen in low pressure ices apart from the contribution from the zero-point vibrations.
