solubility
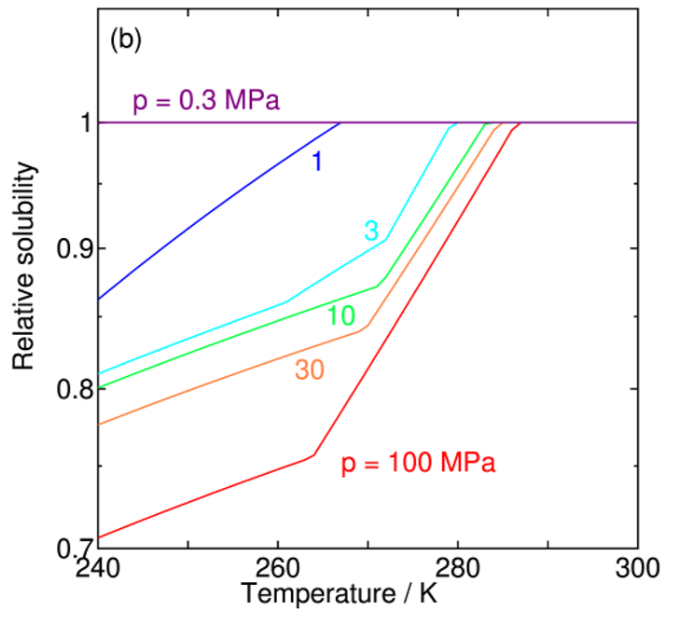
Solubility of Water in Carbon Dioxide
A new paper from our group has been published. This paper investigates the solubility of water in liquid carbon dioxide (CO2) under conditions where water or CO2 hydrate (clathrate hydrate) coexists, using theoretical calculations. The main focus is to quantitatively evaluate the reduction in solubility caused by hydrate formation under low-temperature and high-pressure conditions, and to clarify its temperature and pressure dependencies. This research provides valuable insights into thermodynamic properties related to practical challenges in large-scale CO2 transport, such as pipeline blockage and corrosion in carbon capture and storage (CCS).
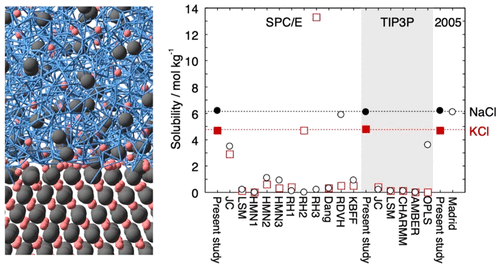
Lennard-Jones Parameters Determined to Reproduce the Solubility of ions
Most classical nonpolarizable ion potential models underestimate the solubility values of NaCl and KCl in water significantly. We determine Lennard-Jones parameters of Na+, K+, and Cl- that reproduce the solubility as well as the hydration free energy in dilute aqueous solutions for three water potential models, SPC/E, TIP3P, and TIP4P/2005. The ion–oxygen distance in the solution and the cation–anion distance in salt are also considered in the parametrization. In addition to the target properties, the hydration enthalpy, hydration entropy, self-diffusion coefficient, coordination number, lattice energy, enthalpy of solution, density, viscosity, and number of contact ion pairs are calculated for comparison with 17 frequently used or recently developed ion potential models.
